Tuesday, 20th May 2025
Chemistry 3 (Practical) (Alternative A) — 09:30am – 11:30am
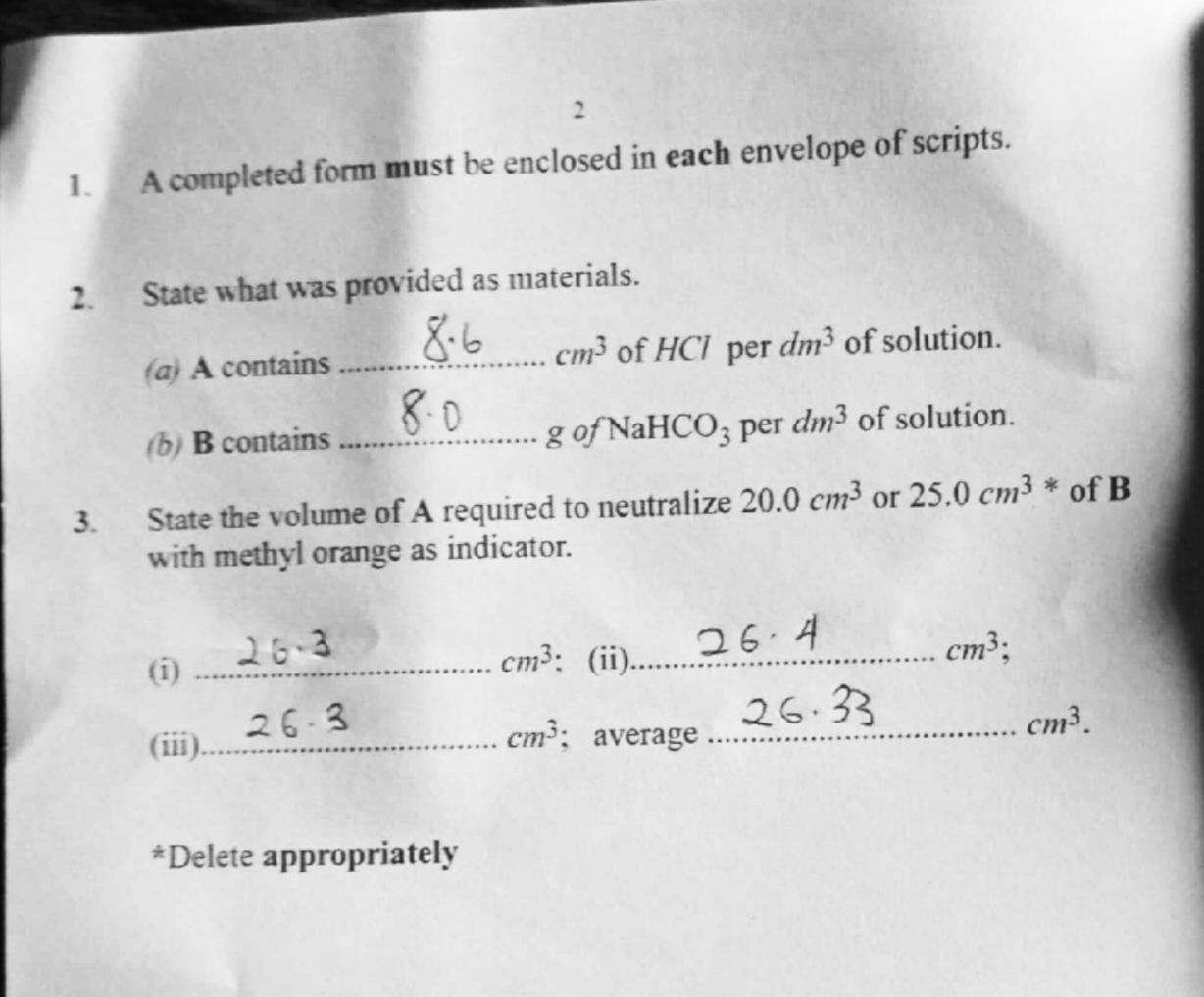
TEACHERS REPORT:
(2)
(a) 8.6
(b) 8.0
(3)
(i) 26.3
(ii) 26.4
(iii) 26. 3
Average 26.33
VERY IMPORTANT – PLEASE READ CAREFULLY
For Question 1:
We used 26.33cm³ as our own school’s end point.
You must use your own school’s end point instead.
Anywhere you see 26.33cm³ in our answer, change it to your own and calculate again.
Example:
In 1bii, we used 26.33cm³ to get CB = 0.0952
→ You should use your school’s end point to get your own CB.
Then in 1biii, we used our 0.0952 to calculate and got 8.0
→ You must use your own CB from 1bii to get your own answer in 1biii.
In 1biv, we used the 8.0 from 1biii to get our final percentage
→ You must use your own value from 1biii to get your correct answer in 1biv.
Chemistry students should understand this.
If confused, kindly ask your Chemistry teacher for help.
NOTE:
If your school wants to use our end point (26.33), the Chemistry teacher must agree and submit something close to it… like 26.10, 26.20, 26.30, etc.
(1)

(2)

(3a)
Ammonia gas (NH₃).
(3b)
(PICK ANY ONE)
Dip a glass rod in concentrated hydrochloric acid and introduce it into the gas jar containing R; the formation of white fumes confirms the presence of ammonia gas.
OR
Expose the gas to hydrogen chloride (HCl) gas to observe formation of white fumes
OR
Introduce a glass rod soaked in concentrated HCl acid into the jar containing the gas; the appearance of dense white fumes confirms the presence of ammonia
(3c)
(NH₄)₂SO₄
(3d)
Ammonium tetraoxosulphate(VI)








CHEMISTRY PRACTICAL SPECIMEN
(1) Great care should be taken to ensure that the information given in items 2 and 3 below does not reach the candidates either directly or indirectly before the examination.
(2) In addition to the fittings and reagents normally contained in a Chemistry laboratory, the following apparatus and materials will be required by each candidate:
(a) one burette of 50.0 cm³ capacity;
(b) one pipette, either 20.0 cm³ or 25.0 cm³. (All candidates at one centre must use pipettes of the same volume. These should be clean and free from grease);
(c) the usual apparatus for titration;
(d) the usual apparatus and reagents for qualitative work including the following with all reagents appropriately labelled:
(i) dilute sodium hydroxide solution
(ii) dilute hydrochloric acid
(iii) dilute trioxonitrate (V) acid
(iv) silver trioxonitrate (V) solution
(v) aqueous barium chloride
(vi) aqueous ammonia
(vii) lime water
(viii) red and blue litmus papers
(ix) dilute tetraoxosulphate (VI) acid
(x) Fehlings solution A & B
(e) spatula
(f) filtration apparatus;
(g) one beaker:
(h) one boiling tube;
(i) four test tubes:
(j) methyl orange indicator;
(k) glass rod;
(l) wash bottle containing distilled/deionized water:
(m) burning splint:
(n) watch glass:
(o) bunsen burner/source of heat;
(p) droppers
(q) mathematical table/calculator;
(r) phenolphthalein indicator.
(3) Each candidate should be supplied with the following, where n is the candidate’s serial number.
(a) 150 cm³ of a solution of HCl, in a corked flask or bottle, labelled An’. These should all be the same containing 8.6 cm³ of concentrated HCI per dm³ of solution.
(b) 150 cm³ of NaHCO, solution in a corked flask or bottle labelled ’Bn’. These should all be the same containing 8.0 g of NaHCO3 per dm³ of solution.
(c) One spatulaful of 1:1 uniform mixture of copper (II) tetraoxosulphate (VI) and glucose in a specimen bottle labelled ’Cn’. This must be the same for all candidates
